An antibody drug called lecanemab significantly reduced the amount of amyloid plaques in the brains of clinical trial participants and slowed their cognitive decline. In trials, the treatment slowed cognitive decline by 25 percent, enough to allow participants to enjoy several more months of independent living. This offers hope of conquering a devastating disease that affects tens of millions of people worldwide. Researchers are looking at drug combinations, vaccines and gene therapies to create the next generation of treatments.
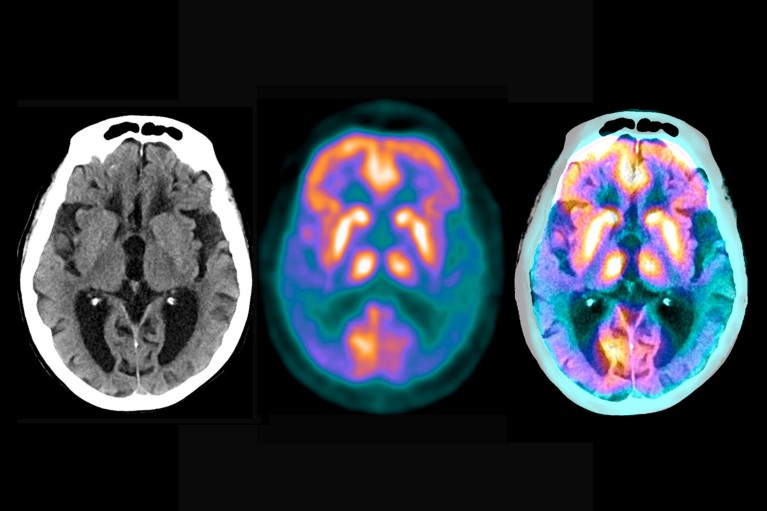
Brain scans reveal the extent of the damage caused by Alzheimer's disease
In December, neurologist Reisa Sperling accepted a lifetime achievement award at an international Alzheimer's conference - for outstanding work on clinical trials for Alzheimer's (AD) treatments.
She created an antibody drug called lecanemab that significantly reduced the amount of amyloid plaques in the brains of clinical trial participants - a telltale sign of the disease - and slowed their cognitive decline. In trials, the treatment slowed cognitive decline by 25 percent, enough to allow participants to enjoy several more months of independent living. This offers hope of conquering a devastating disease that affects tens of millions of people worldwide.
On stage, Sperling clung to the microphone and was in a buoyant mood. She runs a laboratory at Harvard Medical School in Boston, Massachusetts, and has spent more than three long and frustrating decades quietly ploughing her way through Alzheimer's research before finally getting evidence that she and her colleagues are on the right track. "But that's not enough," she said.
Even though it's not perfect, like there's a strict treatment regimen that has to be administered intravenously by a professional caregiver, and the drug can cause potentially life-threatening brain swelling and bleeding, and people who take it have to be monitored regularly, and so on.
Despite these flaws, lecanemab's results offer hope that Alzheimer's may eventually be preventable if treatment is given early enough.
The success also raises another possibility: that the drug and future drugs could be used in combination to tackle different stages of the disease, which are often controlled by different molecules. Few expect monotherapy to solve the problem. But combination therapy trials are expensive and complex because each drug has to be tested individually and with its partners. Pharmaceutical companies may be wary of bundling their product with another in case the combination fails and casts a shadow over their drug.
The increased confidence in the field is not just due to the success of anti-amyloid antibodies. There are also countless potential drugs, and the cupboard is full of potential new treatments -- as well as discarded drugs that are now being cleaned up.
A promising combination
The onset of Alzheimer's disease has a long, silent start. First, plaques -- sticky clumps of beta-amyloid protein -- start to accumulate in the brain. They are soon surrounded by immune cells called microglia, which attempt to nibble at them but ultimately fail.
The plaques grew in size and number, but went unnoticed for years, even decades, until they caused another protein, called tau, to accumulate to toxic levels and scatter around the brain in tangles. The severity of Alzheimer's symptoms correlates with the extent of tau tangles. Current evidence points to tau as the initiator of Alzheimer's symptoms, disability, and eventual death.
So far, individual therapies targeting tau have performed poorly in trials, but scientists believe drugs that destroy tau might work better when used in combination with anti-amyloid therapy.
"We know that amyloid somehow drives the accumulation of pathological tau, and then it spreads through the brain like wildfire," says Bateman, a neuroscientist at Washington University in St. Louis, Missouri. "So we think it makes sense to remove the stuff that's fueling the flames while we're trying to put out the tau tangles fire."

Bateman and his colleagues will test anti-tau antibodies and anti-amyloid antibodies
Bateman and his colleagues began planning such a trial in 2015, but it only recently became feasible because the amyloid therapy proved effective.
Last year, they launched an international trial called Tau NexGen. They are recruiting 168 participants, all of whom are likely to develop Alzheimer's at a young age - usually in their 30s, 40s or 50s - because they have a genetic mutation that causes them to overproduce amyloid-beta. All participants will receive lecanemab and tau reduction antibodies.
Participants were divided into two groups based on whether they had developed symptoms of dementia or expected to develop symptoms within the next decade (these people typically developed symptoms at about the same age as their parents with the genetic mutation).
All participants will receive lecanemab and tau reduction antibodies, but in a different order. Those without symptoms will receive the anti-tau antibody E2814 for a year and then add lecanemab; Symptomatic groups will receive lecanemab for six months and then E2814 will be added. The researchers conducting the trial hope they can use this setup to learn the best combination of treatments.
More anti-tau drugs will eventually be included in studies, with the first trial results expected after 2027.
Tau NexGen is the first, and so far only, ongoing clinical trial of a combination treatment for the condition. A similar US trial 3 is being planned for sporadic, late-onset Alzheimer's disease, which affects the elderly and accounts for the vast majority of cases. The National Institutes of Health (NIH) is expected to decide in the coming months whether it will co-fund the work, called the ATP trial, as a public-private partnership with pharmaceutical companies. If so, hiring could begin next year.
Many pharmaceutical and biotechnology companies are developing anti-tau therapies, some as antibodies, others using other small molecules or newer genetic methods to block the production of pathological forms of tau. Adam Boxer, a neuroscientist at the University of California, San Francisco and co-leader of the ATP trial, said several of the companies have formally expressed interest in participating.
Like Tau NexGen, this will be a prevention trial. Participants had little or no detectable symptoms, but would get evidence from blood tests and scans that their brains contained early signs of plaques and tau tangles. There will be six groups of approximately 900 participants who will receive one of two tau therapies, alone or in combination with lecanemab, lecanemab alone or a placebo.
The research team hopes that anti-tau therapy will enhance the modest benefits of lecanemab - and that, in a virtuous cycle, by reducing plaque burden, lecanemab will create better conditions for anti-tau therapy.
Key to the trial is a series of sensitive new biomarkers - measurements of the brain or blood that can read the state of the disease. Brain scans monitored the presence and severity of amyloid plaques and tau tangles; Blood or cerebrospinal fluid tests measure many other molecules in the pathological chain, such as different forms of amyloid and tau proteins. The researchers expect that the wealth of molecular and clinical data they generate will help reveal more about the mechanisms of Alzheimer's disease. "Current evidence points to tau as the initiator of Alzheimer's disease symptoms, disability and eventual death," Boxer said. "But this hypothesis needs to be tested in humans."
Trials of combination therapies have some drawbacks: they are complex and expensive. Antibodies are expensive treatments on their own. Lecanemab sells for $26,500 for a year's treatment. The original price for a year of Aducanumab was $56,000, but the manufacturer halved the price after a public outcry.
The drugs are also inconvenient for patients because they have to be infused every few weeks. Data from clinical trials suggest that lifelong treatment is needed to stop Alzheimer's. When the infusion is stopped, the disease seems to rebound. Because long-term antibody therapy is impractical, he says, "we think it might make sense to maintain low amyloid status with an oral drug that blocks peptide production once the antibodies clear the plaque."
Such compounds do exist. Starting around 2010, researchers trialled a suite of oral drugs designed to reduce amyloid in the brain by reducing the activity of one of two enzymes, beta-secretase and gamma-secretase, that are key to its production. But clinical trials of these drugs failed 4, and interest dried up -- until they were given a second chance.
Other ingredients
In 2018, a group of drug companies agreed to do something unusual in their usually secretive industry. They decided to share confidential clinical data from six failed trials with each other and with a select group of experts convened by the Alzheimer's Association, a patience-rights lobby based in Chicago, Illinois.
The society hopes to learn as much as possible from disastrous clinical trials, each of which tested a different beta-secretase inhibitor. None of these drugs have shown a benefit - and worse, many have toxic side effects, including, in some cases, cognitive deterioration. Instead of letting the trial data gather dust behind closed doors, the association wants to discuss them in detail. Maria Carrillo, the association's chief scientific officer, said the goal is to "help the field learn more about the disease-related biology that these investigational drugs target."
The panel's 2021 review suggested that the disease in trial participants may have developed too late for such drugs to improve symptoms, and that side effects could have been avoided at lower doses.
Aisen believes it should be possible to use these drugs at low doses to stop plaque recurrence -- once the existing plaque has been cleared by antibodies.
A number of clinical trials targeting gamma-secretory enzymes have also failed. But rather than give up on that goal, the researchers have been working on a more subtle approach. Rather than completely blocking the enzyme - a blunt instrument that causes the toxic side effects observed in the trial - they hope to change its behaviour.
One such regulator, a compound developed in an academic collaboration, will be tested in early-stage clinical trials this year. The drug, which can be taken orally, causes enzymes to cut amyloid into shorter proteins that are non-toxic and can even be protective. The trial will be sponsored by Acta Pharmaceuticals, a startup based in Boston, Massachusetts, and funded by the NIH.
Most of the drugs being considered for combination trials target amyloid or tau proteins. But there are early approaches aimed at improving the brain's natural immune defenses against Alzheimer's. Again, researchers have learned a lot from families with genetic mutations that predispose them to Alzheimer's.
The mutation in question is in a gene called TREM2, which produces a molecule that sits on the surface of the brain's immune system warrior microglia cells. "Tweaking microglia cells could make them more effective at clearing plaques or preventing the spread of amyloid pathology," says neuroscientist Christian Haass of Ludwig Maximilian University in Munich, Germany, "especially if plaque load is first reduced by anti-amyloid therapy." He is planning experiments in mice to test how antibodies that bind TREM2 and activate microglia cells might work if taken in conjunction with anti-amyloid therapy. A similar antibody is in early clinical trials as a standalone treatment.
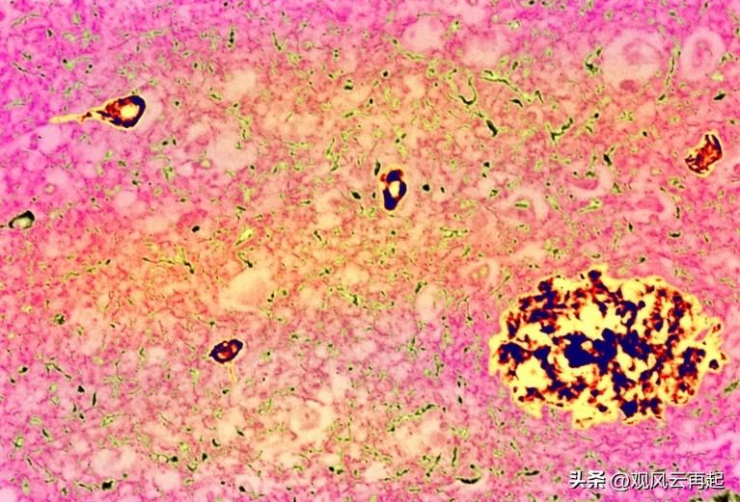
Typical Signs of Alzheimer's Disease: Plaques (large chunks) and tau tangles (small chunks)
Vaccines, genes and blood
More ways to stop Alzheimer's are entering clinical trials. The goal is to deliver useful molecules to the brain through vaccines, viral vectors or blood transfusions.
Like enzyme drugs, vaccines are being reinvented after the first clinical trial of an anti-amyloid vaccine was halted in 2002 after brain inflammation was observed in some participants.
Many pharmaceutical and biotechnology companies are developing additional anti-tau therapies, some as antibodies, others using other small molecules or newer genetic methods to block the production of pathological forms of tau.
There are several anti-tau and anti-amyloid vaccines in the pipeline or in early clinical trials. They contain fragments of tau protein, or amyloid-beta, which are selected and packaged to avoid a severe inflammatory response. They are designed to stimulate the brain's immune system to recognize and destroy complete versions of proteins, and are primarily used to prevent disease or slow the progression of early disease. Scientists are even trying to develop vaccines that attack both tau and amyloid-beta.
Other researchers are betting on gene therapy to conquer Alzheimer's, which is caused by genetic mutations. But gene therapy isn't for everyone, because mutations are known to determine only a small percentage of Alzheimer's cases. But the concept of alternative medicine has been adopted by others. Alkahest, based in SAN Carlos, California, has completed a small clinical trial to test whether factors in the blood of young people can replace those lost in aging.
It is too early to say which, if any, of these potential new treatments will be successful. Most researchers agree that treatment needs to be personalized: individuals at different stages of the disease need different treatments. "It's great to see so many viable approaches being adopted," Eisen said. "But we still have a long way to go."
Still, Sperling said, the range of options that passed clinical testing is encouraging. "Our new light of success is driving us forward."
Article from: Health community


 Contact Us
Contact Us
 Return
Return